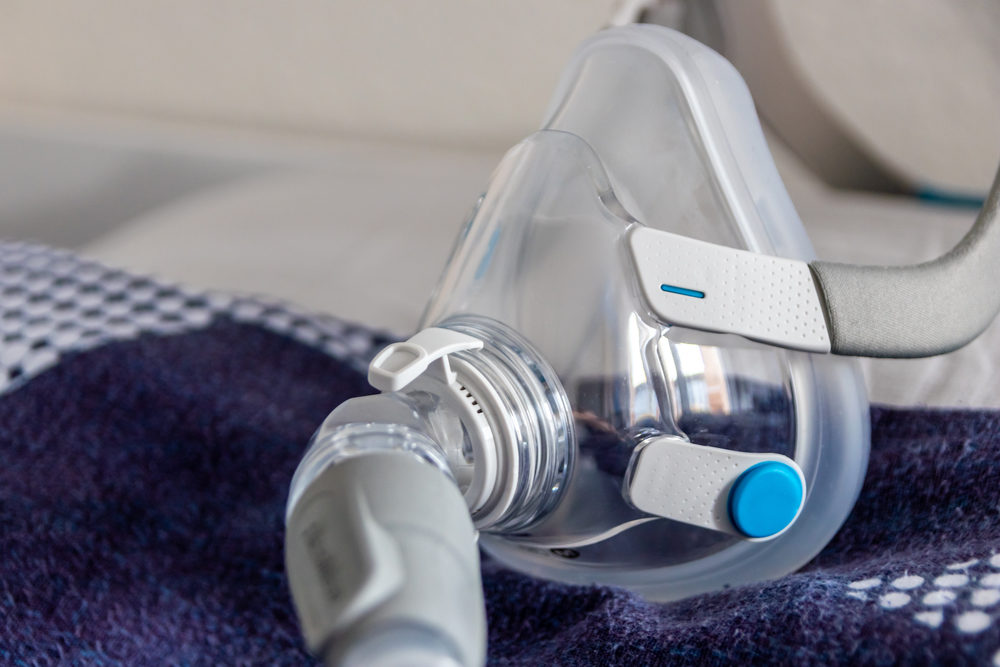
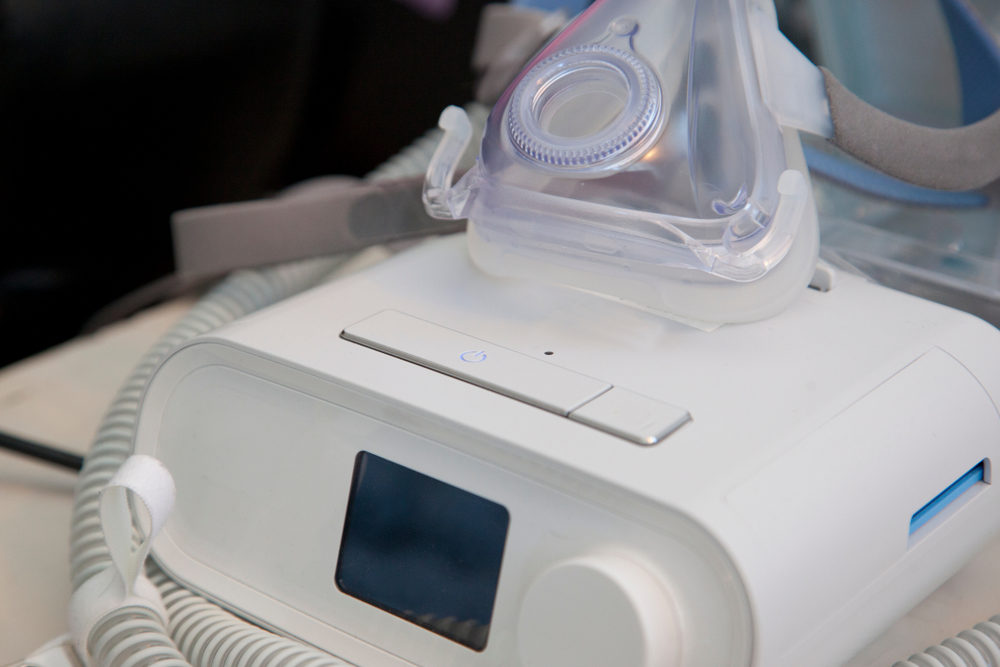
Plaintiffs Seek Compensation for Faulty CPAP and SoClean Devices
An Arkansas man recently filed a new Philips CPAP lawsuit in the Circuit Court of Jefferson County, Arkansas. He claims that after using the company’s DreamStation CPAP machine, he suffered from serious injuries. On the very same day (March 10, 2022), a Michigan man filed…