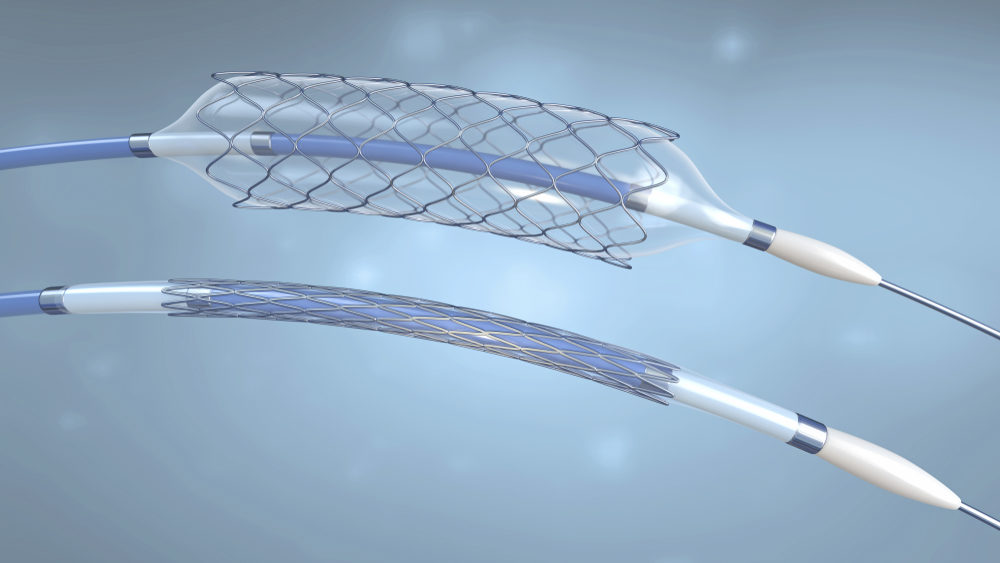
Medtronic Recalls All Valiant Navion Thoracic Graft Systems After Reports of Injuries
Medtronic recently announced in an Urgent Medical Device Recall that it was recalling all unused Medtronic Valiant Navion Thoracic Graft Systems around the world. The company informed physicians to immediately cease use of the device until further notice. Medtronic Recalls Graft System After Receiving Reports…