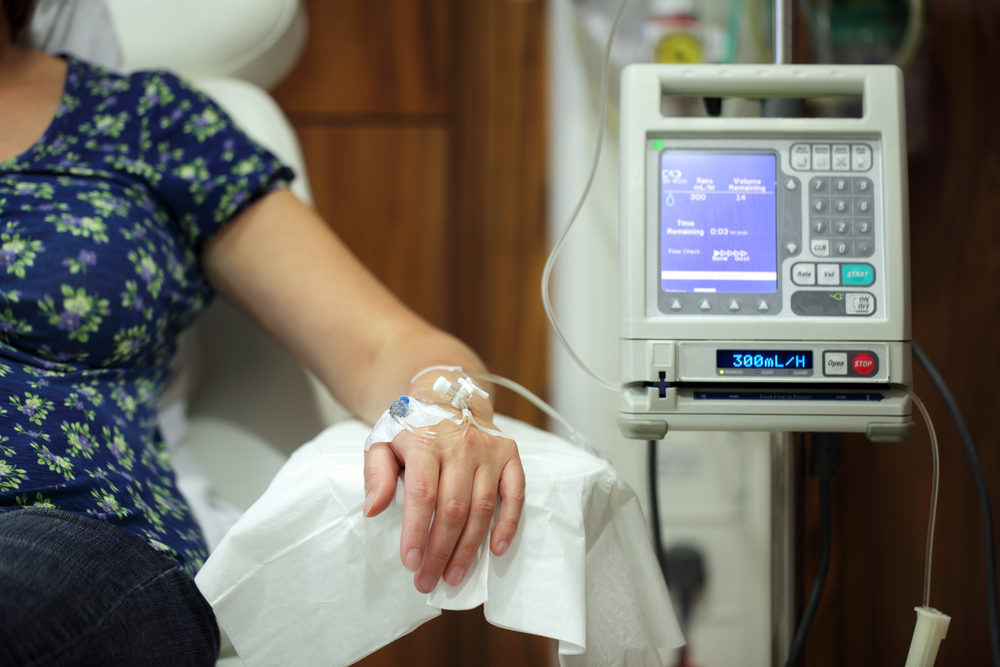
Injectafer manufacturer Daiichi Sankyo is doubling down on their efforts to make more money with their injectable iron supplement, Injectafer, despite the fact that they are missing vital warnings about Injectafer’s safety risks. Considering the company’s history of enticing doctors to use their products, it…
According to a recent CNN report, manufacturer Bayer paid millions to doctors to entice them to use Essure, their permanent birth control device. The news channel analyzed federal data and found that between “August 2013 and December 2017, Bayer paid 11,850 doctors $2.5 million related…
The FDA approved Injectafer (ferric carboxymaltose) in July 2013 for the treatment of iron deficiency anemia in adult patients. The product is injected directly into the bloodstream, where it can quickly replenish iron levels and relieve symptoms. Injectable iron supplements are preferred to oral pills…
The U.S. Consumer Product Safety Commission (CPSC) recently announced a recall of about 9,700 Rollerblade helmets that are primarily used in schools’ physical education programs. These helmets failed to meet a federal safety standard, and pose a risk of head injury. Consumers are advised to…
The FDA received over 26,000 medical device reports related to Essure between November 2002 and December 2017. The reports indicated problems with the permanent birth control device, including migration, breakage, dislocation, and malpositioning. Manufacturer Bayer, despite recently stating they would no longer sell the device…