Just three months after the FDA restricted sales of the permanent birth control product Essure, the manufacturer, Bayer, has decided to halt sales of the device in the U.S. Bayer had stopped selling Essure in all countries other than the U.S. back in September 2017, but had continued to market the product here until recently.
According to the Washington Post, Bayer announced on July 20, 2018 that it would halt sales of Essure by the end of 2018, allegedly based on a decline in U.S. sales of the product. Bayer denied that the move had anything to do with the long-running controversy over the product’s safety and efficacy.
Women Report Serious Essure Side Effects
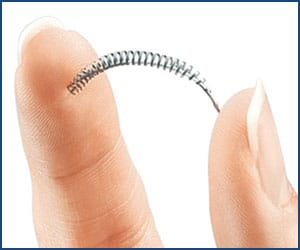
Essure is a permanent form of birth control or female sterilization that works by blocking off a woman’s fallopian tubes. It consists of two small spring-like metal coils made of nickel, steel, and other ingredients that are designed to create a reaction inside a woman’s body that leads to the buildup of scar tissue.
Doctors implant the device via a procedure called “hysteroscopic sterilization.” It requires no incisions and is considered a minimally invasive procedure. Doctors thread a catheter and tiny camera through the vagina and cervix, then insert the devices through that tube. Once the coils are in place, they create scar tissue that over a period of about three months, builds up to block off the tubes, preventing sperm from reaching the eggs.
Despite the relative ease of the device’s placement, thousands of women have complained about serious side effects associated with the device after it is implanted, and Bayer is now defending thousands of lawsuits from alleged injuries from the device.
FDA Warns About Essure Side Effects
The FDA approved the device in 2002, but since 2015, they have been investigating reports of problems associated with it. Between 2002 and December 2017, the FDA received over 26,000 medical device reports related to Essure.
Women described side effects including abdominal pain, heavier menstrual periods and menstrual irregularities, headaches, fatigue, and weight fluctuations. They also reported problems with the device itself, including migration, breakage, organ perforation, malpositioning, and allergic reactions. Sometimes, these problems caused so many complications that the women had to have the devices surgically removed.
In 2016, the FDA required a new, updated “black box warning,” which is the most serious type of warning, to be placed on the product label, “to help to ensure women receive and understand the benefits and risks of this type of device.” The FDA also approved a new “decision checklist” containing key information about the device that doctors were supposed to share with women considering implantation.
When the FDA learned that not enough doctors were using this checklist to inform women of the risks, they decided to take it a step further. In April 2018, they announced that they were restricting sales of Essure only to those doctors and medical centers using the discussion checklist as instructed. From that point on, doctors selling the product were required to go over the checklist with patients, and to have the patients sign it before implantation.
Thousands of Women Seeking Damages in Essure Lawsuits
Bayer attributes the drop in sales of Essure to a decline in the number of women using permanent contraception, an increased reliance on long-acting contraceptives, and on “inaccurate and misleading publicity.”
Women negatively affected by the product started a Facebook group called “Essure Problems” that now has 37,000 members. Essure lawsuits filed in the state of California have been consolidated in Alameda for pre-trial proceedings.








