November is National Alzheimer’s Disease Awareness Month and in an effort to fight this debilitating disease that affects an estimated 5.7 million Americans, Chaffin Luhana LLP team members, Roopal Luhana and Justin Joseph interviewed Dr. Daniel Alkon.
Dr. Daniel Alkon is the President and Chief Scientific Officer of Neurotrope Bioscience, a biotechnology company seeking to develop cures for neurodegenerative and neurodevelopmental diseases. He earned his M.D. from Cornell University. He was laboratory chief at the Neurological Institute of the NIH and founding Scientific Director at Rockefeller Neuroscience Institute.
Can you tell us about your background and your current role at Neurotrope?

I’m trained as a physician, and I also did graduate work in physical chemistry and biochemistry. I was, even as a medical student, very interested in the role of memory in neurologic and psychiatric disorders.
Given my interest and my background, I thought that the best way I could contribute would be to work on the fundamental molecular foundations of memory storage.
Memory storage really involves a paradox. The paradox is how can you, with biological material, record information for the lifetime of an individual? It might last 70, 80 years.
I organized a multidisciplinary team at the NIH’s Institute for Neurologic Disorders and Stroke. We looked for mechanisms associative of memory storage that would be relevant to humans.
After working at the NIH for about 30 years, the Rockefeller family invited me to start a new institute called the Blanchette Rockefeller Neuroscience Institute, which would be devoted to Alzheimer’s disease and disorders of memory.
This was the perfect opportunity to see to what extent the animal mechanisms we’d identified could be applied to humans. We discovered that new structures are actually formed when we form memories, and these new structures depended on some of these molecular pathways that we had previously identified.
Furthermore, when we used drugs to activated the key pathways, we found that we could accelerate learning and memory in animal models. With that, we generated a portfolio of about 150 issued patents and we created a for-profit subsidiary called Neurotrope Bioscience.
I became the Chief Scientific Officer. A couple years later, I was asked to be President of this company as well. I’m also the Scientific Director of a companion company called Neurodiagnostics, which uses some of the same molecular cascades as early biomarkers for Alzheimer’s disease.
What kind of research is Neurotrope currently pursuing?
We have been validating our biomarkers by using autopsies. We actually did a study with the Harvard Brain Bank using frozen samples from Alzheimer’s patients and healthy controls. We received them within two and a half hours of demise.
We analyzed for our primary molecular target, which is called protein kinase-C epsilon (PKCe). We found that when levels of PKCe went down, the disease hallmarks went up, and vice versa. This led us to a whole strategy which we now have as a platform of treating neurologic disorders.
Basically, we are restoring the synaptic networks.
I know that sounds a little bit futuristic, and if you had asked me 15 years ago I would have said it is impossible. But I have no doubt now that it’s possible. We prevented neuronal death and regenerated the synaptic networks.
In doing so, we actually restore the cognitive functions that have been lost. This worked in both animal models of degenerative brain disorders including Alzheimer’s Disease, Stroke, Fragile X, and a series of clinical trials.

We started with a drug called Bryostatin, which was provided to us by the National Cancer Institute. They had isolated this drug for treating cancer, but it didn’t end up working for that purpose.
We treated a series of patients with very advanced Alzheimer’s disease. Some of these patients could not swallow or speak. We restored major functions to these patients.
Once we saw that this was possible, we decided to do a national clinical trial. We did a study with 26 hospitals across the country. We found that these patients were showing improvement of a kind that hadn’t been seen before.
The drug was not just slowing down the cognitive decline, but actually causing cognitive improvement. Several months ago, we launched another national trial to confirm this data. We’ve seen already that this could represent a major breakthrough for treating Alzheimer’s disease.
What makes Bryostatin different from other Alzheimer’s drugs?
We are not just trying to treat the symptoms or slow the disease. Instead, we’re actually trying to reverse the disease and restore the pathways that have been lost.
When we looked at the brains of the animal models, we see that the networks have been restored, and the neurons that were dying are revitalized. That is what gave us encouragement to move the drug to clinical trials.
So far, what we’ve seen is consistent with the animal models. The patients are not just slowing down their decline, they’re actually reversing that decline. Their cognitive function is improving over their baseline.
One of the exciting results is what happened when we stopped treating them.
We stopped treating the patients after 11 weeks and then we monitored them for the next 30 days. The patients’ improvement not only continued, but it actually got better.
Whatever we’ve done is persistent. To me, the only reasonable explanation is that we’ve changed the brain structures. It wouldn’t persist if we were just changing certain neurotransmitters.
What are the next steps for Bryostatin?
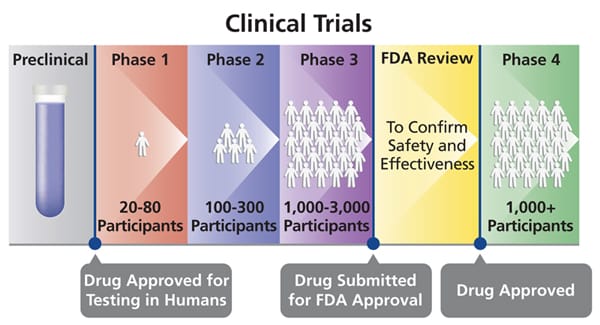
There’s a lot more to be done, of course. We want to extend this kind of therapy out for many more months and see how far we can get the patients to improve.
But what we’re doing right now is to nail down the result we’ve already observed by replicating it with even more patients. We need to show that we have a dose that’s absolutely safe and effective. If we can do that, I think the pharmaceutical industry actually may start to believe that they’d better look in different directions.
Then the question is, what do we do next? That will partially depend on our interactions with the FDA. If they agree that this is a potential answer to an unmet medical need, they may give us some form of an accelerated path, into clinical application.
When you have something that might help patients now, it may not be absolutely essential that we do a full phase three clinical trial. We are hoping that the FDA might allow us to start treating patients right away while we work on more advanced trials.
Is there any agreement in the medical community as to what might cause Alzheimer’s disease?

No, there is no agreement. We don’t know what causes the disease. Most of the pharmaceutical industry has taken the position that the amyloid plaques are causing the loss of synapses and therefore causing the disease.
There’s a reason for their taking that position. Some years ago, it was found that about 3% of Alzheimer’s cases are determined by three genes: presenlin-1, presenlin-2, and amyloid precursor protein. If you have a mutation in one of those genes, you almost certainly will develop Alzheimer’s disease.
If you take those mutations and put them in a mouse, that mouse will produce amyloid plaques. This led to the assumption that amyloid plaques were the cause of Alzheimer’s disease.
However, if you look at autopsies, many Alzheimer’s patients don’t have huge amounts of plaque, particularly at the beginning of the disease. In fact, many of our oldest old fully functional patients have an abundance of amyloid plaques and neurofibrillary tangles, but no Alzheimer’s.
In my opinion, the drug companies have used models that do not sufficiently approximate the disease. If you have huge amounts of amyloid plaques in the mouse brain, and then you treat them and get rid of the plaques, you make the mouse better.
But that’s not what happens in a human with Alzheimer’s. In an Alzheimer’s brain, you start to have some plaques, but the key is when you start to lose synapses. That’s the elephant in the room.
Pioneers like Bob Terry at the University of California San Diego and Paul Coleman at the University of Rochester have looked at the relationship between cognitive dysfunction with the presence of the amyloid plaques.
They found almost no correlation. There was very little relationship between the level of deterioration and the presence of those plaques. But there was a direct strong correlation with the number of synapses. The fewer synapses you had, the stronger was the cognitive decline.
Is there any crossover between Alzheimer’s disease and chronic traumatic encephalopathy (CTE)?
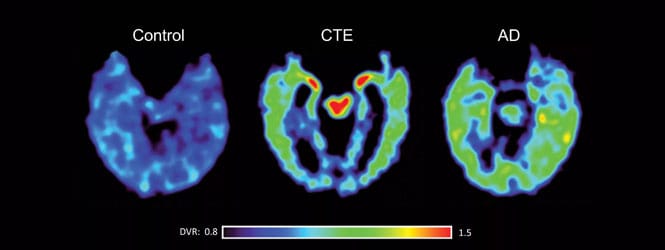
CTE is very interesting. Many patients who have been in athletics or in military circumstances where they’ve had a significant traumatic brain injury. You do see a lot of neurofibrillary tangles and some amyloid.
Unfortunately, this has not been studied in a rigorous systematic way. There have been multiple anecdotal findings, but it’s never been really nailed down as a distinct entity.
There’s no doubt that if you have significant head trauma, you can get the destruction of neurons, destruction of connections, encephalopathy, and many terrible syndromes including suicidal syndromes.
It’s related, but it’s not identical to Alzheimer’s disease. However, I don’t know that it’s been studied systematically enough to know to what degree these are the same or different.
We do know that when patients have Alzheimer’s and when they later have a head trauma, such as from a fall, typically the Alzheimer’s gets worse. But that still doesn’t mean that’s what is causing the disease. What we do know is that there is a spectrum of causes, not one cause but a spectrum.
How does the research funding for Alzheimer’s disease compare to other diseases?
Alzheimer’s disease doesn’t have the level of funding that it should have.
We have about 5.7 million people with Alzheimer’s disease just in the United States. By 2050, that number will grow to 14 million.
It costs nearly $300 billion every year to take care of Alzheimer’s patients in the U.S. If we don’t develop a drug to treat these patients, in 2050 it’s going to be economically unbearable for us. We have to figure out a cure, but there is not enough research funding.
What made you decide to study Alzheimer’s in a way that deviates from mainstream research?
If my point of view were the mainstream, in my opinion we would be making much more rapid progress. The mainstream is still focused on antibodies for amyloid plaques.

A company like Biogen, which is exploring antibodies similar to those of that Johnson & Johnson with 10 years ago, has a $50 or $60 billion market cap. Every time they see a little bit of a blip of improvement, they get another $10 billion.
That’s part of the reason why I became the President of this company. I help raise the money for our research by talking to investors and getting people to look at the facts.
We’ve raised about $70 million, which is just a drop in the bucket. If we really had our druthers, we’d be doing three or four other trials right now in parallel.
Long ago, I decided that every day that I had was a blessing. I saw people suffering as a young medical student. I said, “What days I have left, I’m going to use in the most sincere, dedicated way that I can.”
If I see something that is working, I’ll go along with it. But if I see something that is not working, I’m not going to adhere to that, even if it means I suffer for it, because I’d rather live true. I’ve followed that my whole life.
I feel that I’ve been amazingly blessed for it, because it’s led to very interesting prospects, but I also live on the edge. I always know that I can be snuffed out. Aside from just passing, on I can be overwhelmed by other people’s opinions.
So far that hasn’t happened. I’ve had wonderful experiences with very prominent folks who are willing to listen. There are not that many, but they listen and they say, “This really might make a difference. Keep it up.”
That’s encouraging, but it’s hard. It’s hard to speak against the wave of thinking that is popular and that is commonly accepted.








