Janssen Pharmaceuticals recently announced the recall of one lot of Ortho-Novum 1/35 birth control pills and two lots of Ortho-Novum 7/7/7 birth control pills. The problem is a misprint in the instructions included with the packages, which could cause women to take the pills in the wrong order. That could lead to breakthrough bleeding or an unintended pregnancy.
Janssen Provides Correct Instructions on Website
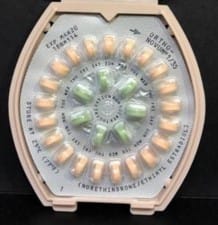
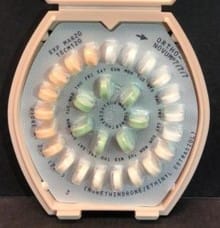
To find out if your pills are affected, look for lot numbers 18BM114 and 18CM120. All products had an expiration date of March 2020. Only the three lots mentioned contained the incorrect instructions. Other Ortho birth control pills are not affected by this recall. All wholesalers, distributors, and pharmacies were told to return the affected products by mailed letter.
Consumers in possession of recalled products can find the correct instructions on Janssen’s website or talk to their doctors if they have questions. They can also call Janssen at 1-800-526-7736 Monday through Friday from 9:00 a.m. to 8:00 p.m. Eastern Standard Time.
The FDA advised women not to stop taking the pills, and to follow the correct instructions if they miss a dose. The pills themselves are safe if used correctly. Instead of following the incorrect instructions, women should first take the 21 active peach or white/peach-colored pills for three weeks, and then take the green inactive pills for one week.
Taytulla Birth Control Also Affected by Packaging Errors
A similar packaging error led to the recall of Taytulla birth control pills on May 29, 2018. Manufacturer Allergan announced it was recalling one lot, which included nearly 170,000 packs after a physician notified them of the problem.
In the affected packs, the inactive pills were placed first in order, rather than last, as they should have been. The pills were color-coded, but women new to the product or who took the pills without carefully checking the packaging would be at risk for contraceptive failure. If women took them in the order provided, they would be at risk for unintended pregnancy.
To check for the recalled products, women should look for lot number 5620706, and an expiration date of May 2019. The affected packages came on the market in August 2017.
Women who discover that they have the recalled product and think they may have been injured as a result should keep the recalled product in a secure dry place and contact a lawyer immediately.
Women File Lawsuit Seeking Compensation for Unintended Pregnancies
In 2015, over 100 women filed a birth control negligence lawsuit in Philadelphia against Endo Pharmaceuticals and other companies that made or distributed birth control pills under various brand names. The packages were rotated 180 degrees, which reversed the order of the pills. Women unknowingly took the placebo pills first, believing they were the active pills. All but four of the women became pregnant after using birth control pills that were packaged out of order. The women sought millions in damages for lost wages and child-rearing expenses.








