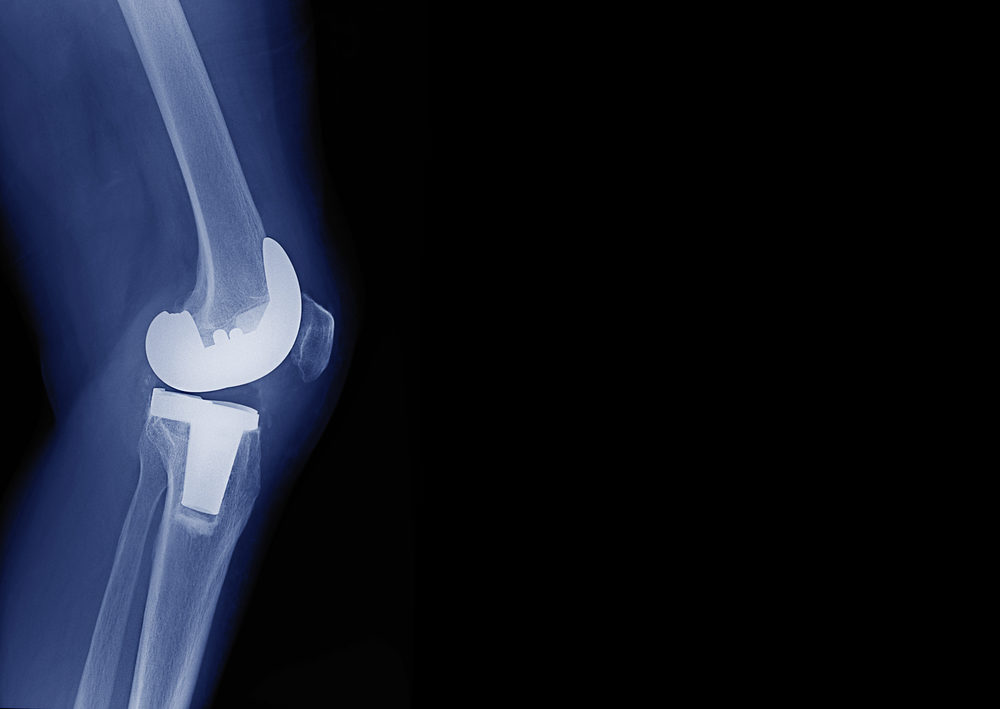
New York Man Receives Two Exactech Knee Implants and Both Fail
On June 11, 2022, a New York couple filed a new Exactech lawsuit against the makers of the Exactech Optetrak Total Knee System. Doctors implanted the system into one of the plaintiffs, and he claims that he later suffered from serious injuries. He seeks compensatory…